ABOUT US
ABOUT


We are dedicated to the goal of preserving a person’s function and quality of life into old age. Our drug attacks neurodegeneration at its roots
QR Pharma is a specialty pharmaceutical company developing novel treatments for Alzheimer’s, Parkinson’s and other neurodegenerative diseases. QR is attacking neurodegeneration at its roots with its product candidate Posiphen® which inhibits the production of brain toxic proteins.
QR Pharmaceuticals develops, manufactures, and distributes essential medications with a focus on regulatory compliance, clinical relevance, and real-world accessibility. We operate within a strictly defined quality system built on GMP protocols, with every product batch tracked from raw materials to final shipment. Our production is driven by therapeutic demand and practical application, not by trend or overextension. Our facility specializes in high-volume generics and select branded formulations, all registered under national and international health authorities. The core of our model is operational clarity. From stability testing to batch release, every process follows a single logic: no shortcuts, no guesswork, no missing data. That’s how we maintain continuity across regions and market conditions. QR Pharmaceuticals supports both institutional clients and licensed distribution networks. Hospital systems, government buyers, and authorized wholesalers work directly with our regulatory and logistics teams to maintain consistent supply flow. Our catalog reflects formulary standards across multiple therapeutic areas, with particular strength in cardiovascular, anti-infective, endocrinology, and pain management.
Manufacturing Standards and QA Control
We don’t separate production from responsibility. Each product line is overseen by qualified pharmacists, analytical chemists, and regulatory staff embedded in the process itself. Every dosage form is validated according to pharmacopeial specifications, and analytical records are maintained for full traceability. All QA procedures are conducted on-site. That includes incoming material testing, in-process controls, and post-production analysis. Our methods comply with USP, BP, and NOM standards, depending on market destination. The lab uses HPLC, GC, and FTIR methods with calibrated equipment and audit-ready documentation. Release decisions are based strictly on pre-defined acceptance criteria, not forecast pressure or delivery schedules. We also conduct ongoing stability studies in ICH-controlled chambers for both long-term and accelerated conditions. This ensures shelf-life accuracy and product behavior under shipping and storage variables. Every package that leaves our warehouse has been reviewed not only for label compliance, but for consistency with validated shelf-life data and shipment tolerances.
Registration, Market Access, and Global Supply
QR Pharmaceuticals maintains registration dossiers with health agencies across multiple markets, including Latin America, the Middle East, and Southeast Asia. Our regulatory team is experienced in managing full CTD formats, bilingual submissions, pharmacovigilance commitments, and post-market variation filing. In the US, QR Pharmaceuticals works through licensed partners and contract representatives. Products distributed into the US market comply with all FDA importation protocols and are supported by valid Certificates of Pharmaceutical Product (CPP) and GMP site approvals. Every export batch is documented with analytical results, COAs, and shipping records in compliance with FDA, DEA (where applicable), and customs requirements.
International distribution includes:
- Finished dosage export under approved site licensing
- Private-label formulation and contract manufacturing
- Supply integration with public health and humanitarian procurement systems
We maintain strict control over routing and temperature-sensitive handling where required. Our shipping documentation includes real-time temperature monitoring for flagged SKUs and photographic packing logs at departure and arrival.
Research Focus and Pipeline Planning
Our product pipeline is built through collaboration with hospital-based pharmacologists and community care physicians. We prioritize drugs based on therapeutic gaps, off-patent opportunities, and real-use data, not lab theory. Our formulation group conducts compatibility testing with excipients commonly used across geographies to ensure broader tolerability. While our core is generics, we allocate R&D resources toward improving release kinetics, combination therapy formats, and dose reduction where possible. Every new formulation goes through accelerated pilot runs before scale-up. We do not release compounds that lack robust manufacturing feasibility or clear prescriber utility.
Recent development projects include:
- Fixed-dose antihypertensives with reduced pill load
- Oral pain relief formulations with extended-release matrix
- Compact dosing strips for pediatric antipyretics
- Improved bioavailability versions of essential antifungals
Each of these projects is tracked in a locked protocol system with full documentation of formulation steps, performance data, and stability outcomes. Our internal documentation structure mirrors international site audit expectations and allows clear validation trails for any formulation history.
Who We Work With and How We Operate
QR Pharmaceuticals operates through clearly defined commercial relationships. Our clients include government agencies, regional pharmacy chains, and hospital procurement networks. We only work with licensed distributors, and all downstream partners must provide active certification for local trade. We handle procurement through structured agreements. That includes raw material forecasting, volume-based pricing, and contract-linked delivery schedules. Our system is designed to minimize disruption in supply chains, especially in essential drug categories where outages carry clinical consequences. We do not run open-market listings or accept unscheduled tenders. Our entire commercial model is based on documented need, regulatory validation, and structured allocation.
Final Note on Operations
QR Pharmaceuticals exists to manufacture and deliver medications that serve actual treatment paths. We operate within a framework of clinical logic, documented standards, and complete regulatory visibility. Our role isn’t to fill catalogs with options. It’s to produce and supply drugs that physicians use, that patients need, and that regulators approve. Every service we provide connects directly to that purpose.
DISEASE FOCUS
OUR FOCUSNeurodegenerative diseases are the largest unmet need for our aging population, our economy and the world. QR Pharma understands how brain cells die in both slow chronic & fast acute injuries to the brain and the nervous system
Alzheimer’s Disease (AD) A type of dementia that causes problems with memory, thinking and behavior. Symptoms usually develop slowly and get worse over time, becoming severe enough to interfere with daily tasks.
Parkinson’s disease (PD): A chronic and progressive movement disorder, meaning that symptoms continue and worsen over time. It involves the malfunction and death of vital nerve cells in the brain, called neurons.
INNOVATION
INNOVATIONAttacking Neurodegeneration at it’s Roots
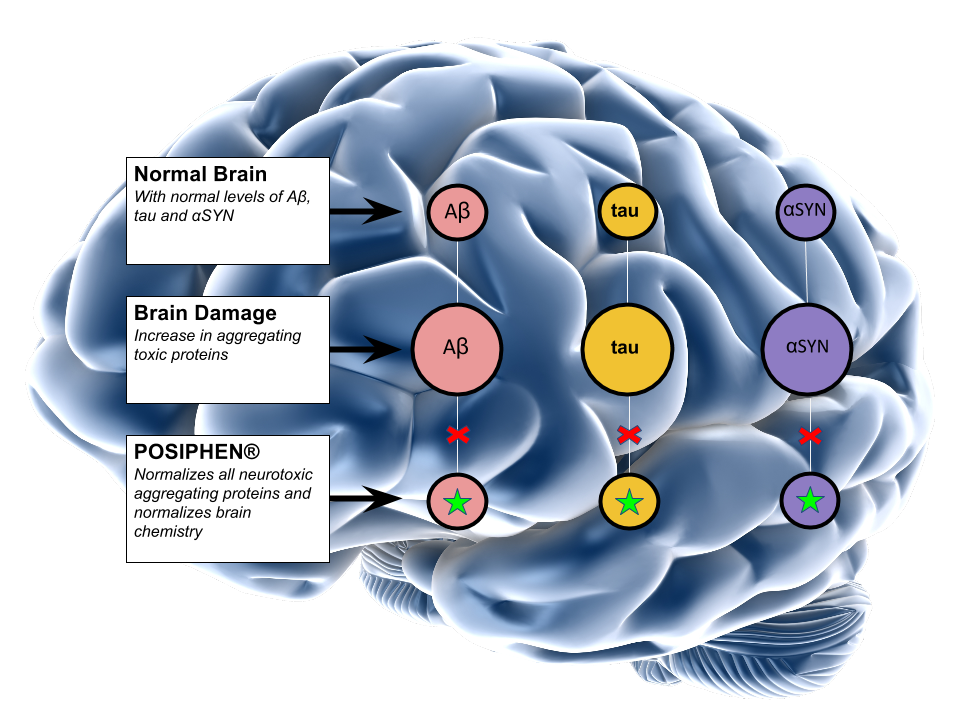
Posiphen®
QR Pharma’s lead compound, Posiphen® inhibits synthesis of amyloid precursor protein (APP), Tau and α-Synuclein. Posiphen® is distinct from other Alzheimer’s disease (AD) drugs currently in development, because it inhibits the formation of toxic proteins before they are formed
Our Pipeline
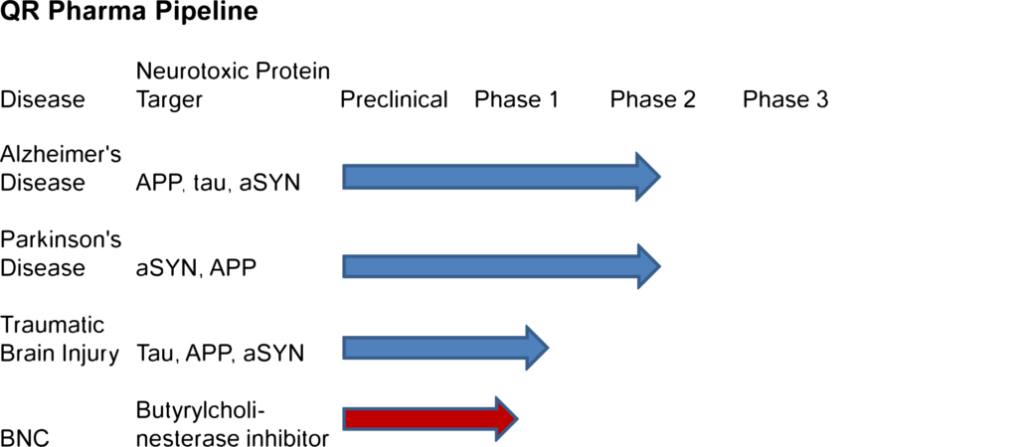
- Alzheimer’s disease: QR is starting a 1 month phase IIa study in mild to moderate Alzheimer
patients in June and expects the data to be available at the beginning of 2017 - Parkinson’s disease: human safety was completes
- Traumatic brain injury: human safety was completed
PRESS ROOM
BEHIND THE SCIENCEWhat is happening at QR Pharma
Press Release
Maria Maccecchini to Receive 2011 Iris...
 Maria Maccecchini to Receive 2011 Iris Newmann Award – November 16, 2011 Article >>>>>
Maria Maccecchini to Receive 2011 Iris Newmann Award – November 16, 2011 Article >>>>> News Articles
Healthcare Businesswomen’s Association: Women in Science...
 Healthcare Businesswomen’s Association: Women in Science – Winter 2011
Healthcare Businesswomen’s Association: Women in Science – Winter 2011 Scientific Presentations
Targeting Alzheimer’s and Other Neurodegenerative Diseases...
 Speaker: Maria L. Maccecchini Presentation, November 30, 2010. CNDR Talk: University of Pennsylvania, More >>>>>
Speaker: Maria L. Maccecchini Presentation, November 30, 2010. CNDR Talk: University of Pennsylvania, More >>>>> Scientific Publications
NeuroScience Network – Is Alzheimer’s Disease...
 NeuroScience Network – Is Alzheimer’s Disease Close To Being Cured? – May 11, 2010
NeuroScience Network – Is Alzheimer’s Disease Close To Being Cured? – May 11, 2010