Posiphen® is orally available, well behaved and well qualified as an oral development candidate.
Three clinical studies have been conducted with Posiphen®. The key findings from the three clinical studies are highlighted below.
Single ascending dose (SAD) in 60 healthy volunteers vs. placebo
- Drug determined to be safe, with an NOAEL of 80 mg and an MTD of 160 mg
- Dose limiting toxicity was nausea
Multiple ascending doses (MAD) in 48 healthy volunteers vs. placebo
- Drug determined to be safe at an NOAEL of 240 mg/day (60 mg QID)
- PK: Drug absorbed rapidly; Cmax = 1.5 hrs, T1/2 = 5 hrs in plasma
- At doses up to 60 mg QID, Posiphen® showed no symptoms indicative of inhibition of either acetyl- or butyrylcholinesterase
Proof of mechanism of action (MOA) in 5 Mild Cognitive Impairment (MCI) patients
- Concentrations of Posiphen® in the brain, extrapolated from blood and CSF, were 8x higher than in plasm
- TTmax in plasma = 5 hours in CSF > 12 hours
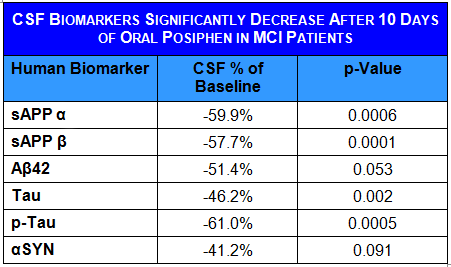
- Ten days of treatment with Posiphen® normalized CSF levels of sAPP, and Tau, and reduced αSYN and a series of inflammatory markers
- PK of Posiphen in brain suggest that doses of 20 to 40 mg once a day could achieve desired effect
- These data are reported to the FDA in SN 0016 and SN 0018
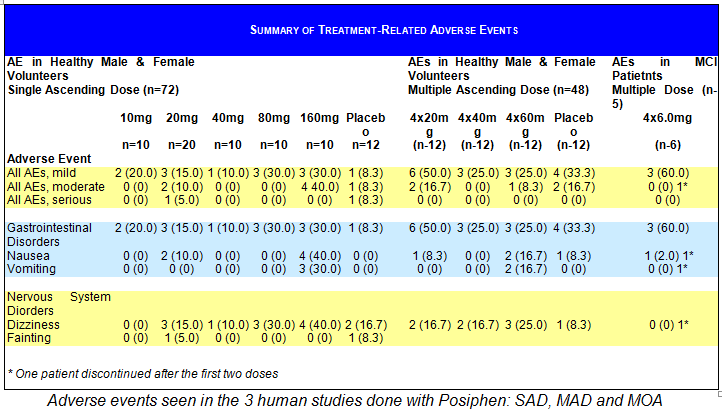
Summary of Safety in Humans from all three Studies
Posiphen®’s pattern of AEs was similar to that seen in typical studies in healthy normal volunteers, with an overall incidence of 33.3% among placebo-treated subjects and 35% for all Posiphen® treatment groups combined. In the single ascending dose study the group given the highest dose of 160 mg/day showed 31.7% AEs that were treatment-related. In the multiple ascending dose and in the MOA study there was no dose response to the adverse events. Most AEs were of short duration, mild or moderate in severity, resolved without medical intervention, and occurred in one or a few subjects. Only two subjects experienced severe AEs, including symptoms associated with orthostatic hypotension (1 placebo and 1 POSIPHEN® 20-mg subject).
The Human POC Study
 5 patients with mild cognitive impairment were treated for 10 days with Posiphen® with the safe dose of 4×60 mg, (240 mg/day). CSF and plasma were drawn over 12 hours on day 0 and day 11. Levels of Posiphen® and metabolites were measured in plasma and CSF over the 12 hours. Posiphen® PK in plasma corresponded to what the Company had seen in the previous clinical safety studies: T1/2 = 5 hrs. In CSF, however, Posiphen® showed a much longer half-life of T1/2 > 12 hours. QR conducted an identical experiment in rats, where it is possible to measure the PK of Posiphen® in plasma, CSF and brain. By taking the human plasma/CSF and rat plasma/CSF/brain levels QR was able to extrapolate to the human brain levels and calculate them to be eight times higher than plasma levels. This is consistent with the data the Company has in mice, where in a number of studies, Posiphen® levels were found to be 8-9 times higher in brain than in plasma.
5 patients with mild cognitive impairment were treated for 10 days with Posiphen® with the safe dose of 4×60 mg, (240 mg/day). CSF and plasma were drawn over 12 hours on day 0 and day 11. Levels of Posiphen® and metabolites were measured in plasma and CSF over the 12 hours. Posiphen® PK in plasma corresponded to what the Company had seen in the previous clinical safety studies: T1/2 = 5 hrs. In CSF, however, Posiphen® showed a much longer half-life of T1/2 > 12 hours. QR conducted an identical experiment in rats, where it is possible to measure the PK of Posiphen® in plasma, CSF and brain. By taking the human plasma/CSF and rat plasma/CSF/brain levels QR was able to extrapolate to the human brain levels and calculate them to be eight times higher than plasma levels. This is consistent with the data the Company has in mice, where in a number of studies, Posiphen® levels were found to be 8-9 times higher in brain than in plasma.
Posiphen®’s extended presence in the brain is matched by an extended effect, reducing levels of αSYN, sAPP and tau for the whole period tested (over 12 hours). The extended effect of Posiphen® in humans was consistent with the preclinical data in rodent brains.
The persistence of Posiphen® in the CSF and brain and the extended pharmacodynamic effect observed make Posiphen® a good candidate for QD dosing. Extrapolated brain levels of Posiphen® at 60 mg QID were far in excess of levels required to down-regulate APP and αSYN. The regulation of translation across the toxic aggregating proteins is similarly responsive to Posiphen®, suggesting similar dosing in AD, PD and HD. QR further compared Posiphen® brain levels of mice that showed full reversal of their AD or PD behavior and calculated that the optimum brain levels to achieve full efficacy are between 150 and 500 nM. Using three different extrapolation/comparison calculations the Company deduced that a daily dose of 20-60 mg should achieve desired brain levels and efficacy.
 MCI patients treated for 10 days and extracted CSF for 12 hours before and after the last administration of Posiphen® show about 50% lower levels of aggregating toxic proteins; the levels measured have dropped to levels found in healthy normal volunteers.
MCI patients treated for 10 days and extracted CSF for 12 hours before and after the last administration of Posiphen® show about 50% lower levels of aggregating toxic proteins; the levels measured have dropped to levels found in healthy normal volunteers.
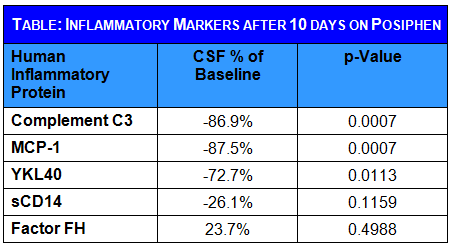
MCI patients also show high levels of inflammatory factors and microglia activation factors in their CSF. Posiphen® significantly lowered the levels of a number of inflammation factors in their CSF
New Phase IIa Study in Alzheimer Patients
In collaboration with ADCS (Alzheimer’s disease Cooperative Study) QR is starting a phase IIa study in mild to moderate Alzheimer’s patients.
3×12 Alzheimer patients are to be treated for one month with 2 doses of Posiphen® and placebo. The endpoints are: APP and Abeta levels in spinal fluid; other biomarkers in spinal fluid; inflammatory markers in spinal fluid; SILK (stable isotope labeling kinetics) in spinal fluid with synthesis and degradation rates; cognition and safety.
In order to obtain all the above endpoints patients will have one spinal tab at the beginning of the study and a 36 hour spinal fluid collection at the end. For the cognitive outcome they will be tested at the beginning and the day before the 36 hr spinal fluid collection at the end.
While Alzheimer’s disease is the most common disease among the elderly and the most lucrative market, the large body of preclinical data accumulated to date opens the potential of wider applicability of Posiphen® in other neurodegenerative disorders.
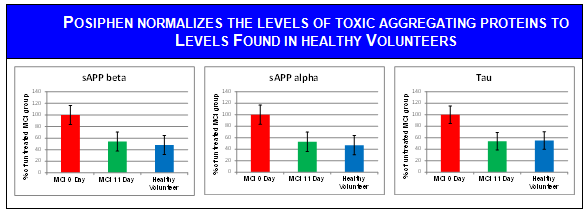
Aggregating proteins measured in the CSF of MCI patients in the MOA study described above. POSIPHEN® given for 10 days normalizes APP and Tau
