ATTACKING NEURODEGENERATION AT IT’S ROOTS
Posiphen® is a small, hydrophobic, orally available molecule that enters the brain readily. It is the only drug ever described that inhibits more than one neurotoxic aggregating protein.
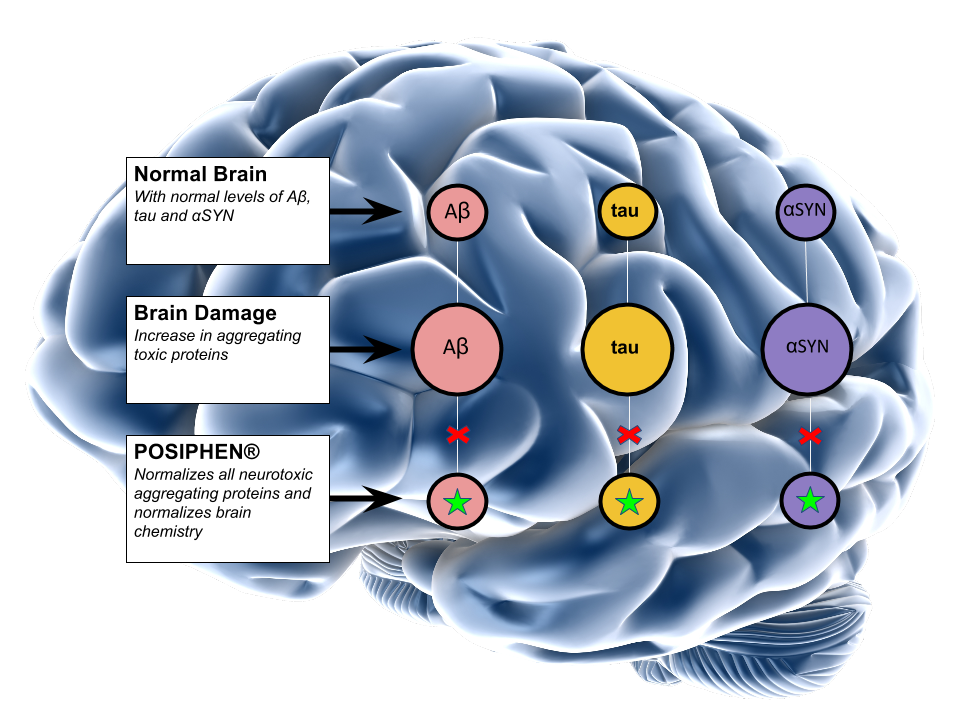
- Brain insults enhance synthesis of several neurotoxic aggregating proteins, such as Aβ, tau and αSYN
- Traditional Alzheimer’s and Parkinson’s approaches aim to remove single neurodegenerative proteins (e.g. Aβ for AD or αSYN for PD)
- QR Pharma’s lead compound, Posiphen® inhibits synthesis of amyloid precursor protein (APP), tau and α-Synuclein; it is distinct from other Alzheimer’s disease drugs currently in development, because it inhibits the formation of several toxic proteins, rather than removing individual toxic protein after they are produced.
- Posiphen® normalizes expression of several neurotoxic aggregating proteins by potentiating the binding and/or activity of an inhibitory protein, IRP1, to the shared conserved regulatory motif, IRE
MECHANISM OF ACTION
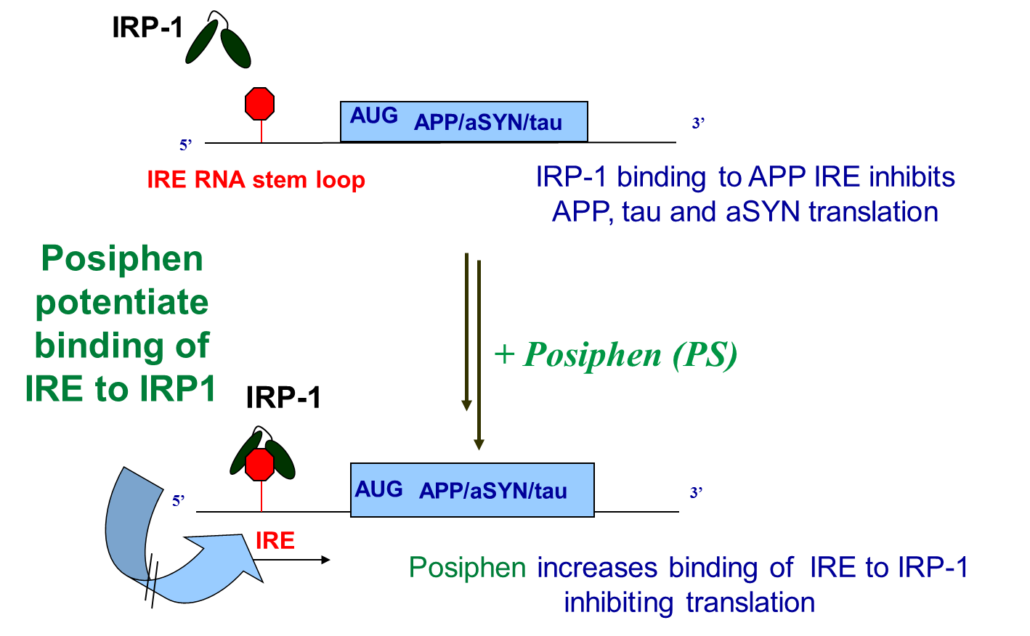
Posiphen® enhances the binding of the IRP1 inhibitory protein to a common regulatory sequence on mRNAs coding for neurotoxic aggregating proteins
Neurotoxic Aggregating Proteins are involved in a series of neurodegenerative diseases and share common features
- Protein misfolding and aggregation underpin many common neurological and systemic diseases. On the neurological side, this diverse group of diseases includes Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), transmissible spongiform encephalopathies (TSEs) and amyotrophic lateral sclerosis (ALS). Despite obvious differences in the neural cell population impacted, clinical symptoms and disease progression, these disorders share common features and regulatory elements that are relevant to the mechanism of action of Posiphen®.
- Neurotoxic aggregating proteins such as APP, αSYN and tau share similar features relative to gene activation, protein synthesis, folding and misfolding, toxicity and aggregation, as described in Disease Focus:
mRNA translation of neurotoxic aggregating proteins is up-regulated by iron (Fe) and down-regulated by iron regulatory protein-1 (IRP1)
Posiphen® interferes with this second step of the common cascade of the aggregating proteins. It enhances the binding and/or activity of IRP1 to the iron response element (IRE) stem loop in the 5’UTR of the mRNAs of neurotoxic aggregating proteins, therefore specifically lowering their synthesis. By potentiating the IRE/IRP1 complex, Posiphen® lowers the level of free mRNA to be translated by the ribosome.
QR Pharma studied the mechanism of action by a total of four approaches to confirm the mechanism and to understand specificity of binding.
- RNA pulldown inhibition and shows that IRP1 specifically binds to IREs of neurotoxic aggregating proteins and that Posiphen® ipotentiates the binding of the IRE to IRP1, therefore, lowering protein translation. – Conducted by Jack Roger’s laboratory at Massachusetts General Hospital
- Thermophoresis binding of cloned purified IRP1 to synthetic IREs of neurotoxic aggregating proteins shows that Posiphen® specifically binds to the IRE/IRP1 complexes of neurotoxic aggregating proteins and increases the stability of the complexes, therefore, lowering protein translation. – Conducted by Rodrigo Vasquez in Prem Reddy’s lab at Mt. SinaiHospital
- Proteomics, to explore the breadth of Posiphen®’s effect on protein inhibition i. The data very clearly show that Posiphen® inhibits neurotoxic aggregating proteins very specifically. – Conducted in by Carlos Barrera in Salim Merali’s lab at Temple University
- Activity in vitro and in vivo, measure inhibitory effect of Posiphen® on neurotoxic aggregating proteins and on unrelated proteins in tissue culture cells, mouse brains and human spinal fluid.
CHH, et al., J Biol Chem, 2010
Rogers JT, et al, JNT, 2011
Mikkilineni S, et al, Parkinson’s Disease, 2012
POSIPHEN® inhibits enhanced translation of neurotoxic aggregating proteins, such as APP, Tau and αSYN. By acting on more than one toxic protein it hence holds promise to change the course of Alzheimer’s and Parkinson’s disease.
QR Pharma, 2016
POSIPHEN® AND ALZHEIMER’S DISEASE (AD)
β amyloid (Aβ) is a hydrophobic, neurotoxic self-aggregating 40 to 42 amino acid peptide that accumulates preferentially within senile plaques in the brain and is the target of many therapeutic clinical trials.
Aβ, C31 and N-APP are neurotoxic fragments derived from Amyloid Precursor Protein (APP). Beyond the ample literature around Aβ, tau protein has also been implicated in the pathogenesis of AD.
By normalizing the expression of APP, Posiphen® interferes with a basic pathway that leads to dementia in Down Syndrome, AD and likely other conditions, such as head trauma and stroke.
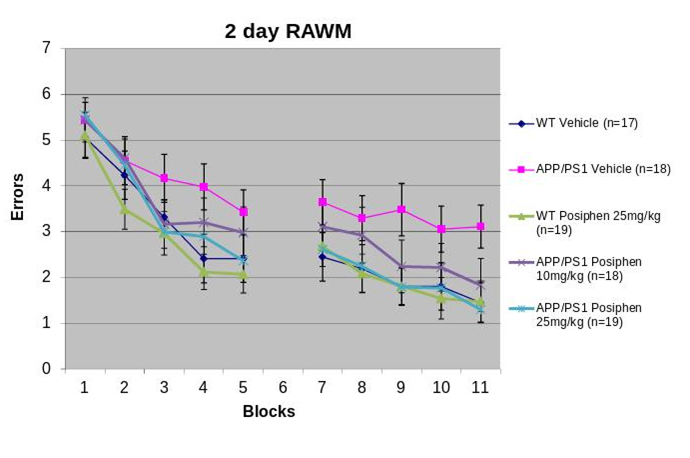
POSIPHEN® AND PARKINSON’S DISEASE (PD)
It is characterized by progressive motor and non-motor decline that substantially contributes to disability and loss of quality of life. There is no treatment that blocks the neurodegeneration and progression of the disease.
A large body of evidence indicates that α-synuclein (αSYN) is involved in the pathogenesis of PD and is a valid target for developing drugs to treat this disease. αSYN mutations and gene multiplications are known to cause familial PD and Lewy bodies, which are a neuropathological hallmark of PD, consist of aggregated αSYN in all PD patients, including the sporadic forms.
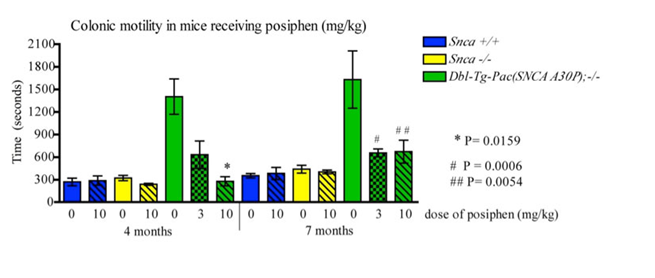
POSIPHEN® AND TRAUMATIC BRAIN INJURY (TBI)
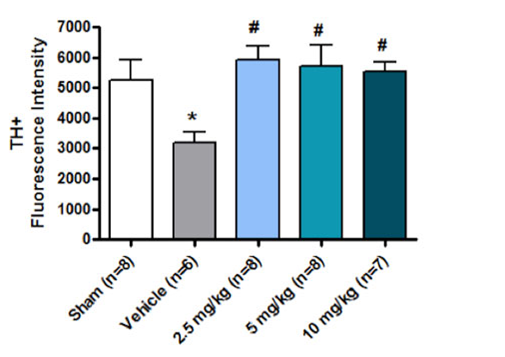
Rats were subjected to fluid percussion injury (FPI) or sham operation to one side of the brain and Posiphen® was administered 1 hour post injury. Three doses of Posiphen® plus saline were given intraperitonealy for 4 weeks. At the termination of the treatment, all the rats were sacrificed and sections of the brain were stained with tyrosine hydroxylase (TH), wherein TH stains only live cells. The amounts of TH immunoreactivity in the whole striatum of the brain slices were measured.
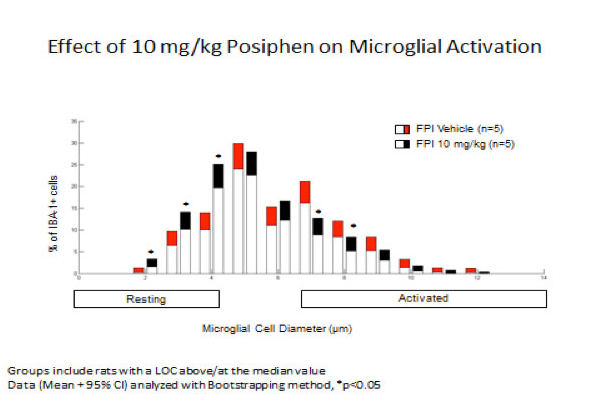
Because FPI can induce microglial activation, we checked whether Posiphen® would reverse this pathological. Microglial activation was assessed by quantitative measure of the diameter of IBA-1-positive cells (ionized calcium adaptor binding protein). Microglia with cell body diameters less than 5 µm had a resting morphology characterized by multiple ramified processes. Hyper-ramified microglia/partially activated microglia had a mean cell body diameter of 5-6 μm. Fully activated amoeboid microglia had a mean cell body diameter of 7-14 μm.
<0.05. and the vehicle group was compared with the Posiphen®-treatment groups with one-way ANOVA, Bonferroni comparisons, wherein #p<0.05. In the Posiphen® group, the rats treated with 3 doses of Posiphen® showed a statistically significant increase over the vehicle treated animals in the number of surviving cells in the ipsilateral area of the brain at all 3 doses. Posiphen® protected the striatum in fluid perfusion injury at all 3 doses tested.