QR Pharma, Inc. Presents New Data on Posiphen in Traumatic Brain Injury at 2016 Military Health System Research Symposium–Posiphen Reverses Behavioral Deficits and Neuropathology Caused by Traumatic Brain Injury–Berwyn, Pa., August 17, 2016 — QR Pharma, Inc., a privately held Phase 2 biopharmaceutical company developing novel therapies for the treatment of Alzheimer’s, Parkinson’s and other neurodegenerative diseases, today announced the presentation of non-clinical data relating to its lead clinical product candidate, Posiphen, at the 2016 Military Health System Research Symposium (MHSRS) being held August 15 – 18 in Orlando, Florida.
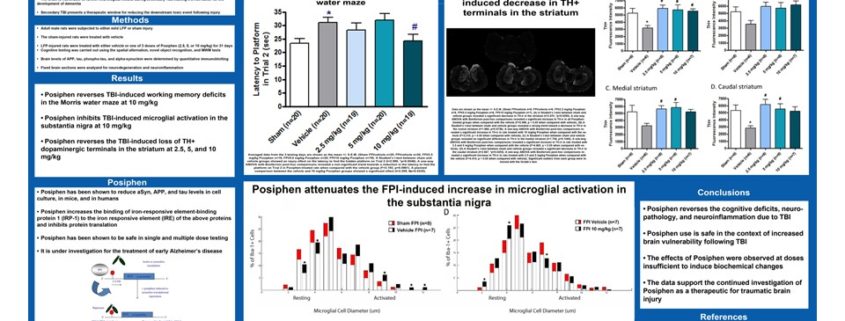
The presentation highlighted studies conducted by investigators from The David Geffen School of Medicine at UCLA characterizing the effects of Posiphen in a non-clinical model of traumatic brain injury (TBI). These blinded studies revealed that Posiphen (2.5, 5 and 10 mg/kg) administered daily attenuated spatial working memory deficits while also rescuing tissue damage, normalizing inflammation and reversing the molecular pathology caused by TBI.
“These are impressive results for Posiphen in a high-fidelity rodent model of TBI,” commented Marie-Françoise Chesselet, M.D., Ph.D., Professor and Interim Chair of the Department of Neurology at UCLA and principle investigator for the studies. “Posiphen completely rescues working memory and consistently improves cell function and survival in the substantia nigra and striatum, regions of the brain that are often damaged following repeated TBI leading to the onset of Parkinson’s disease.”
“These studies provide further confirmation of the neuroprotective effects of Posiphen,” stated Maria Maccecchini, Ph.D., President and CEO of QR Pharma. “These results add to a deep data set supporting the unique activity and potentially broad application of Posiphen in TBI and other neurodegenerative conditions, such as Parkinson’s and Alzheimer’s.”
A full copy of the MHSRS poster entitled, “Posiphen reverses behavioral deficits and neuropathology in a rat model of mild traumatic brain injury,” can be accessed by visiting the “Scientific Presentations” in the Press Room section of QR Pharma’s website at http://www.qrpharma.wbprg.pw.
About QR Pharma, Inc. Headquartered in Berwyn, Pennsylvania, QR Pharma, Inc. is a clinical-stage specialty pharmaceutical company committed to developing therapeutics with novel approaches for the treatment of cognitive impairment in neurological disorders. QR is currently developing Posiphen as a disease-modifying drug for acute as well as chronic neurodegeneration and BNC for advanced Alzheimer’s disease. For more information on QR Pharma, please visit the company’s website, http://www.qrpharma.wbprg.pw
Contact: Maria Maccecchini
Tel: 610-727 3710
Fax: 610 727 4001
maccecchini@qrpharma.com
http://www.qrpharma.wbprg.pw